There are more than 500,000 medical devices on the market today. These range from everyday items, like syringes and gloves, to high-tech implants and AI-driven diagnostics. And each year brings a surge of new devices and improvements; the US FDA authorized 124 new medical devices in 2023, an annual record high.
Medical device distributors must stay current with the latest advances to make informed business decisions, help improve patient care, and remain competitive in the healthcare market.
Staying informed about medtech trends is vital for strategic planning, and this article explores how to stay updated and offer your clients the latest technology from around the world.
Contents
Why medical device distributors should (must!) stay informed
New devices often offer better results, less-invasive treatments, or cost savings vs. older ones. Knowing about these advances early lets you bring greater value to patients and providers, while falling behind risks missed opportunities (and sales advantages) or even patient harm when outdated tools are offered in place of newer, safer options.
Medical devices’ complex regulatory environment is another reason keep up. Agencies continually update guidelines to ensure safety and efficacy for new technologies. The FDA’s Center for Devices and Radiological Health (CDRH) is one example. New regulations (e.g., the EU’s Medical Device Regulation) and standards can directly impact which products can be sold and how they must be handled. As a distributor, you have to stay aware of these changes to remain compliant and to anticipate shifts in market demand (such as when older device models are phased out or novel categories receive approval).
How to stay informed and ahead of the medical device landscape
The sheer volume and diversity of new developments can be overwhelming. Innovations span many domains – from improved biomaterials and implants to wearable health monitors, AI-driven imaging software, and beyond. Many of these advances originate from global research and startup hubs, resulting in relevant information being scattered across various journals, conferences, regulatory filings, and industry news sources worldwide.
Distributors must be cautious not only to learn about new devices, but also to vet their safety, regulatory clearance, and market readiness before offering them to customers.
Despite these challenges, there are effective ways to stay current. Here are some key strategies
Industry news and journals
Regularly read well-known industry publications and scientific journals that cover medical technology. Trade journals and news sites (eg., MedTech Dive, MassDevice, or Medical Device and Diagnostic Industry) provide daily news on product launches, regulatory approvals, mergers, and market trends. Subscribing to these can deliver headlines to your inbox each morning. There are also free medical news apps that aggregate updates; for instance, the Medscape MedPulse app curates healthcare news, and websites like MassDevice focus specifically on medical device news.
Academic journals and clinical trial reports provide deeper insights into how new devices perform. Reading summaries or press releases from journals like NEJM, JAMA, or specialty journals can alert you to breakthrough studies (e.g., a new stent that greatly outperforms the old standard).
Regularly scan a mix of news and scholarly sources to develop a broad awareness of what’s new and notable.
Conferences and trade shows
Industry events are one of the best ways to see emerging technologies firsthand and connect with innovators. Major medical device trade shows – such as MEDICA in Germany, which features around 5,000–6,000 exhibitors from over 70 countries each year – showcase a huge range of new products, prototypes, and services under one roof. This is why you’ll find Yasui there every year, introducing the Yasui koplight™ cordless lighted retractor and demonstrating our Japanese standard of quality.
Walk the exhibition floor and talk with company representatives – you’ll discover cutting-edge solutions before they hit wider distribution. Likewise, scientific congresses and professional society meetings (such as those in cardiology, orthopedics, and radiology) often feature sessions on new device research and exhibitor halls with demonstrations.

Try to attend relevant conferences or, at the very least, follow their published proceedings. Even local and regional medical technology workshops or demo days can provide insight into innovations on the horizon. The networking at these events is equally valuable – you can build relationships with manufacturers, startup founders, and clinical leaders, creating a pipeline for ongoing information sharing.
Professional networks and associations
Tap into the knowledge network of your peers. Professional associations related to medical devices or healthcare distribution often provide educational resources, newsletters, and forums for discussion. For example, in the United States, the Health Industry Distributors Association (HIDA) and the Advanced Medical Technology Association (AdvaMed) provide industry reports, webinars, and email bulletins that highlight emerging trends and best practices.
Join and take part in such groups (or their LinkedIn communities) to receive curated updates, and to ask questions and learn from others’ experiences. Online groups and social media can also play a role here. LinkedIn has many medtech and healthcare supply chain groups where members share articles and insights.

Simply following major medical device companies or thought leaders on social platforms will populate your feed with their latest news. Engaging with an industry community keeps you informed and provides context to understand which innovations are gaining traction.
Monitor regulatory and approval announcements
Since new device approvals and safety alerts often signal important changes, keep an eye on communications from regulatory bodies. The US FDA’s CDRH, for instance, publishes “News and Updates” and offers email list subscriptions so that stakeholders can receive alerts on device approvals, recalls, safety notices, and guidance changes.
Set up email alerts or RSS feeds for FDA announcements (or the equivalent agencies in other markets like Europe’s EMA/MHRA) helps you make sure not to miss clearance of breakthrough devices or new compliance rules.
Review summaries of annual reports and public meetings from regulators. These often highlight priority areas (e.g., digital health oversight, post-market surveillance results) that can inform your strategic focus.
Stay informed about the regulatory landscape to anticipate which innovations are truly market-ready and align your offerings with the latest standards.
Digital tools for updates
Today’s information technology can help you keep up without feeling overwhelmed. Consider setting custom Google Alerts for specific keywords (e.g., “medical device innovation”, “FDA approval orthopedics”) so that relevant news is emailed to you as it appears. Many industry publications and consultants also offer email newsletters or weekly digests that compile the top stories; for example, Forbes’s “Innovation Rx” newsletter covers developments in medicine and health tech.
Using an RSS feed reader app to follow multiple blog or news sites in one place can save time. Another approach is to use healthcare news aggregator apps or platforms (some are AI-driven) that learn your interests and surface new content accordingly. Even podcasts and webinars can be valuable sources – subscribing to a well-regarded medtech podcast or tuning into monthly webinars by research firms (like Deloitte or IQVIA) can keep you informed during your commute or downtime. Integrate these digital tools into your routine to create a steady, manageable flow of information that matches your interests.
Continuous learning and training
Maintaining a learning mindset will help you adapt to innovations. Encourage yourself and your team to pursue ongoing education. This could mean attending workshops on new medical technologies, enrolling in short courses or certification programs (for example, in biomedical engineering basics or healthcare IT), or inviting experts to give lunch-and-learn seminars at your company.
Academic and clinical institutions sometimes offer extension courses or online modules on medical device regulation, quality assurance, or technology management. These offerings can deepen your understanding of the field’s direction. And reviewing key scientific literature or even taking part in research collaborations can provide firsthand insight into where innovations are headed.
Keep ahead in the industry to lead the field in sales
In the dynamic medical technology industry, keeping up with innovation is an active, ongoing process. Medical device distributors, clinicians, and other professionals must intentionally stay informed to navigate the constant stream of new products, techniques, and regulations. Regularly engage with industry news, scientific research, and professional networks, and use conferences and digital tools to your advantage.
Staying current helps you make smarter decisions about which emerging devices to distribute or adopt, ensure compliance with the latest standards, and ultimately deliver better outcomes to patients and healthcare partners. The role of protocols in medical device safety
A device distributors love
Our Yasui koplight™ is a big hit with distributors thanks to its made-in-Japan innovation, sleek design, and physician-informed ergonomics.
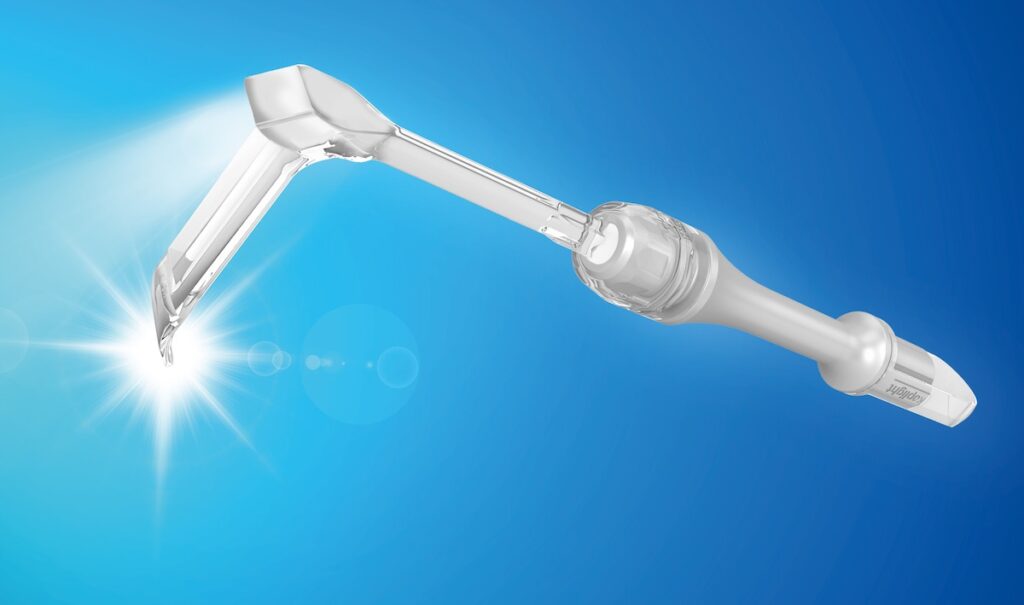
If you’re interested in distributing or purchasing the koplight™, please get in touch with us.

